We use science to move the world towards better health.
Our Science
Transformative Framework for Cellular Therapeutic Development

Discovering cutting edge in vitro models and products to advance technology and streamline cellular manufacturing.

Developing therapeutic cellular applications based on our end-to-end cellular therapy “CryoStem Platform” consisting of “Collection - Processing - Cryobanking - Return to Point-of-Care”.

Delivering Expanded Autologous Mesenchymal Stem Cell Infusion Therapies to the medical community at large and proprietary cellular processing and cryopreservation technologies to the world

Partnering with forward-focused biologics developers to create collaborative relationships to develop and obtain approval of cellular therapy indications (especially for those with high unmet medical needs) and design and deliver new therapies built on our End-to-End “CryoStem Platform”
Our Clinical Pipeline Approach
Our pipeline comprises a balance of wholly-owned and partnered programs. We collaborate with strategic partners when additional resources and expertise may help bring our cellular therapies to patients more quickly.
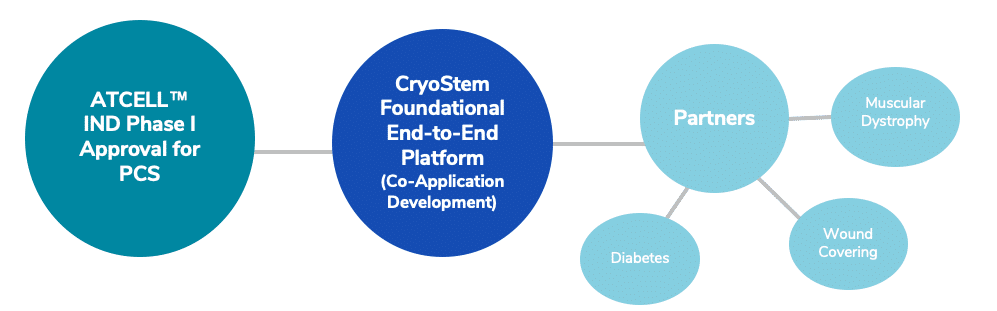
Investigational New Drug (IND) FDA Cleared for Phase I Clinical Trial, September 2020
American CryoStem received FDA clearance by the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) Phase I Clinical Trial “ATCell, a proprietary expanded autologous adipose-derived mesenchymal stem cell therapy for the treatment of Post-Concussion Syndrome (PCS). PCS is a complex central nervous system disorder triggered by one or more concussive injuries. The purpose of the Phase I Clinical Trial is to evaluate the safety and efficacy of ATCell™ infusion therapy. We aim for ATCELL™ to be a valid therapeutic modality to treat PCS and other mild traumatic injuries (mTBI) by offering a cellular therapy option to patients not responding to the current standard of medical care.
Pre-Clinical and Product Development Cooperative Research and Development Agreement (CRADA) for ATCELL™
New Cellular Analyses Metrics and Advanced Therapy Applications
The Research and Development Collaboration CRADA entitled “Stem Cells for Regeneration and Medical Innovation” is a multifaceted and multistaged project centered on:
- Creating in vitro (test tube) assays to standardize and commercialize new treatment protocols
- Optimizing quality control measures
- Developing standardized protocol potency assays for precise therapy dosing
The Collaboration team plans to validate and standardize baseline and assay metrics to identify mesenchymal stem cell (MSC) characteristics and quantities across various cell types and investigate opportunities for testing protocol standardization for biologic drug development. The Collaboration will further investigate the creation of predictive and prescriptive cellular models in support of future FDA Biologic License Applications (BLAs). The Company maintains the rights to commercialize all technology developed under the CRADA Agreement and will endeavor to commercialize these technologies to create scientific and economic value for all stakeholders. This collaboration will be conducted at the Biomedical Research Laboratory in Bethesda, Maryland. CRADA Process
American CryoStem enters into a cooperative research and development agreement CRADA to develop standardized treatment protocols
Pre-Clinical Development for Duchenne Muscular Dystrophy (DMD)
Duchenne Muscular Dystrophy (“DMD”) is a rare, genetic and fatal childhood disease characterized by rapidly progressive muscle weakness and wasting due to degeneration of skeletal, smooth and cardiac muscle It is the most common and severe form of muscular dystrophy among children, afflicting about 1 in 3,500 males at birth.
American CryoStem has paired up with RaceMD®, a 501(c)(3) nonprofit organization, formed in 2009 to accelerate the search for intermediate therapies to prolong the lives and health of children with DMD.
The RaceMD team of physicians and researchers will be collaborating with CRYO’s laboratory staff and scientists to develop a clinical protocol for DMD, harnessing the foundational science and technology of the CRYOSTEM PLATFORM and utilizing the company’s IND clearance for its own IND “safety and efficacy” - without needing to modify the underlying manufacturing, testing or delivery methodology of the ATCELL™ product. This accelerated IND application approach will enable a more expeditious route with the FDA IND approval process.
